[ad_1]
Back in 2014, Omar Yaghi, a chemistry professor at the University of California, noticed something unusual about a new water-attracting material his lab was developing. Pulling water out of the air is useful for a lot of things (think about the silica beads that come in packaging to keep things dry) but drying out desiccants in order to reuse them generally means heating them to very high temperatures, often around 400°F, which uses a lot of energy. But Yaghi’s material, an atomic-scale lattice work replete with billions of tiny pores, known as a metal-organic framework (MOF), was giving up its water at a much lower temperature, around 113°F, equivalent to that of a tepid cup of coffee. “I immediately thought I could take this into the desert, and at night it extracts the water from the air,” Yaghi says. “During the day when it’s hot and sunny I can harvest the drinking water.”
Yaghi, 58, is something of a one-man phenomenon in the world of chemistry. He is known for his work over the past three decades pioneering a field known as reticular chemistry, which involves precisely engineering MOF crystals with molecular-level pores. Those materials can have some interesting properties. If you’re trying to store gaseous CO2, for instance, a container containing a MOF with pores tailored to fit CO2 molecules can actually store more of them than an empty container: the MOF attracts those CO2 molecules like bees to honey. Such materials have dozens of potential applications, from electronic sensors to drug delivery. Yaghi’s work founding and developing the field has made him one of the most cited chemists in the world, and he has been showered with awards and citations for his work, including a nomination for the Nobel Prize in chemistry (his name featured prominently in predictions for the 2022 winner).
Out of all the potential routes for further exploration, though, Yaghi has in recent years been primarily focused on two potential uses for reticular chemistry: fighting climate change, and expanding access to drinking water. In 2020, he started a company called Atoco to carry out those aims (the company’s existence has not previously been reported).
Yaghi is sensitive to how vital drinking water can be. He grew up in Amman, Jordan, in the 1970s, a time when the city only supplied water for a few hours every week or two. As a child, he would wake up early in the morning to wait by the faucet with containers to store a week’s supply of water for the family. After the 2014 experiment, he felt he had the basis for an invention that could free families around the world from that sort of struggle forever. “You can have water harvesters that are operating off-grid anywhere,” Yaghi says. “You have control over your own water. I call it water independence.”
Read more: The Ocean is the Next Frontier for the Carbon Removal Industry
It’s possible today to pull water out of the air (the humble dehumidifier does so, for instance) but Yaghi says that machines making use of MOF materials, with pores precisely tailored to capture water molecules, could produce a steady supply of water with very little energy, and in drier environments than anything available today. They can even produce water using only passive energy from the sun’s heat. In a July 2023 paper published in Nature Water, Yaghi and a group of collaborators demonstrated a material dubbed MOF-303 that was able to produce a steady drip of water in Death Valley, Calif., one of the driest places on earth, just from being left out in the sun. (Technically, Atoco’s process is not absorption but rather adsorption—the former describes when external liquid is soaked up by the entire volume of an object, like a sponge dropped in a bucket of water, whereas the latter is when external liquid adheres to the surface of the object, like when windows mist up when it’s hot and humid outside.)
Yaghi launched Atoco in order to scale up production of reticular materials like MOFs. While Yaghi has worked with other companies attempting to scale up the technology for water harvesting in the past, Atoco says it aims to use newer, more advanced reticular materials, and to develop novel devices like water harvesters that work without electricity (other companies are working to develop electrically-powered water harvesters using reticular chemistry, as is Atoco). Samer Taha, Atoco’s CEO, says that their machines will be able to function in drier environments than anything available today, and with about half the energy on average. Among the devices the company wants to develop is an electrically powered machine the size of a large desktop computer that will be able to produce about 100-200 liters of drinking water per day, Taha says. The company declined to share when the devices will become available.
“This is something that the world needs,” Yaghi says, citing the fact that almost two-thirds of people in the world face severe water scarcity at least one month out of the year. The U.N. is projecting that by the year 2050 almost 5 billion people will be experiencing water stress.

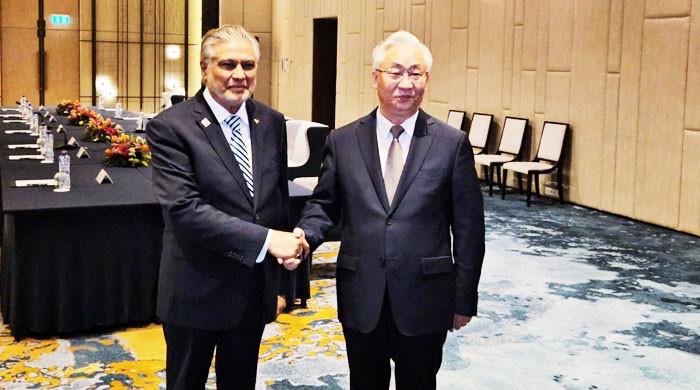
Omar Yaghi and Samer Taha with one of their water harvesting prototypes.
David Huff—Courtesy of Atoco
Yaghi’s technology won’t just be able to draw water out of the air—he says his materials will also be useful for adsorbing carbon dioxide. That could be useful for removing carbon dioxide from industrial smokestacks, or for extracting the greenhouse gas directly from the atmosphere.
Both industries are still in their infancy, though they’ve recently been gaining more traction. Tax credits passed in the Inflation Reduction Act last summer have spurred investment in carbon capture for industrial facilities, and a $1.2 billion investment by the U.S. Department of Energy for two large projects in Texas and Louisiana last month has given the direct-air carbon capture a major boost as well. (No direct air capture facilities currently being planned will be large enough to meaningfully offset humanity’s emissions, though proponents of the technology say it’s important to invest in it now so that we will have facilities that are large enough to make a difference one day.)
Read more: California Sunshine Could be Key to Combating Drought
A major impediment to the carbon capture sector is the tremendous amount of energy that it generally takes to draw CO2 out of the air, or even to filter it in higher concentrations from smokestacks. Most of the carbon capture projects in use today use filters or chemical solutions like potassium hydroxide to trap CO2, but Taha says that using materials made using reticular chemistry could lower that power input substantially. (Other companies are already using reticular materials for carbon capture, but Atoco says that it will be able to offer more effective chemistries and produce them cheaply). “It’s really hard to give a figure now, because we are in the process of building these systems,” Taha says. “But we expect something between 30 to 50% [efficiency] improvement [over today’s standard carbon capture technology], if not more.” It’s a claim that has yet to be borne out. If successful though, that kind of power reduction could make a huge difference in the feasibility of scaling up large-scale carbon capture systems.
Making good on them, however, will be no easy feat. Production of the reticular materials they’re attempting to use has been limited to the laboratory scale, and one of Atoco’s main difficulties will be in trying to scale up that production process. “We don’t claim that from day one, we’re going to make it very scalable, very cheap,” Taha says. “But if we look at the starting materials, they are available and not expensive. So that’s the light at the end of the tunnel.”
Yaghi, for his part, says Atoco is oriented around a social mission. “Quite frankly, originally I was not interested in [starting a company].” Yaghi says. “I wanted to be in the lab. But I’m the inventor of this field of research, and it falls upon me to push these technologies to the next level so that they reach society.”
Correction, Sept. 15
The original version of this story misstated how much of the world experiences water stress. It is two-thirds of the world’s population for at least one month a year, not one-third almost every month.
Correction, Sept. 20
The original version of this story misstated the type of material Omar Yaghi is developing. It is water-adsorbing, not absorbing.
More Must-Reads From TIME
[ad_2]
Source link